The 14 Metabolic Health Biomarkers You Need to Test
High chance you're doctor isn't ordering these
One of the more common questions I get here:
“Phys - what panels should I order to better understand my metabolic state?”
We waste no time.
Here are the 14 biomarkers to keep your metabolic house in order.
Biomarker #1: Lean Body Mass-to-Visceral Fat Ratio
What it measures?
The ratio of metabolically active tissue (muscle) vs. the harmful fat wrapped around your organs.
Why it matters?
LBM/VF addresses the limitations of traditional metrics like BMI - especially for those of us who habitually resistance train.
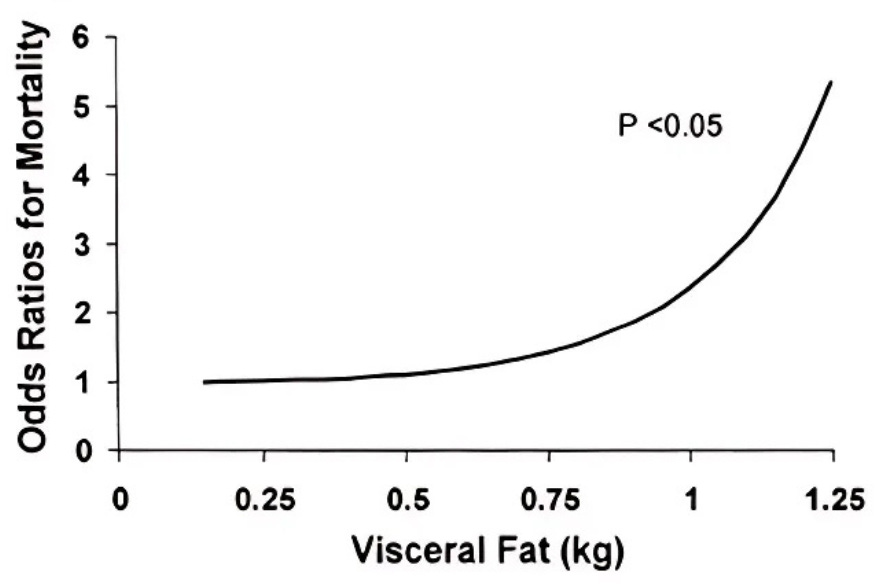
Every 1% in relative muscle mass decreases the risk of metabolic disease by ~20%1. Meanwhile, visceral fat accumulation tracks directly with all-cause mortality2.
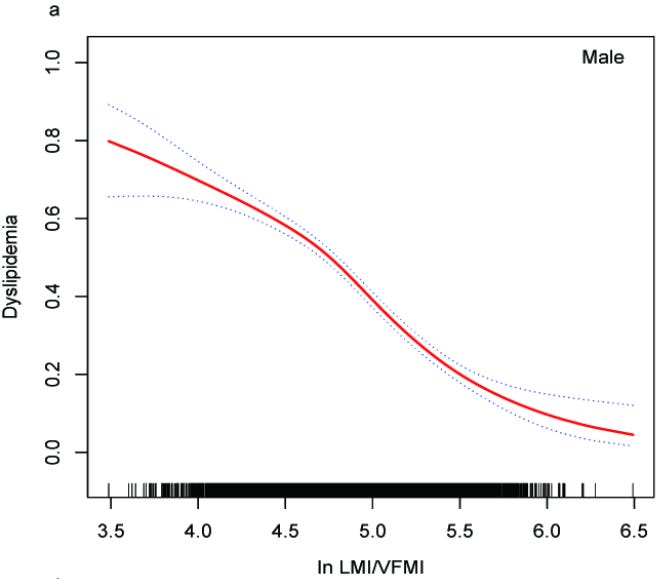
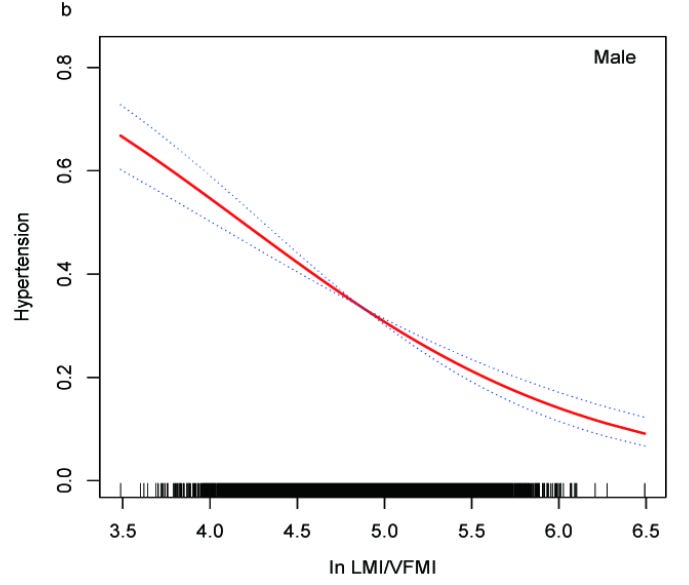
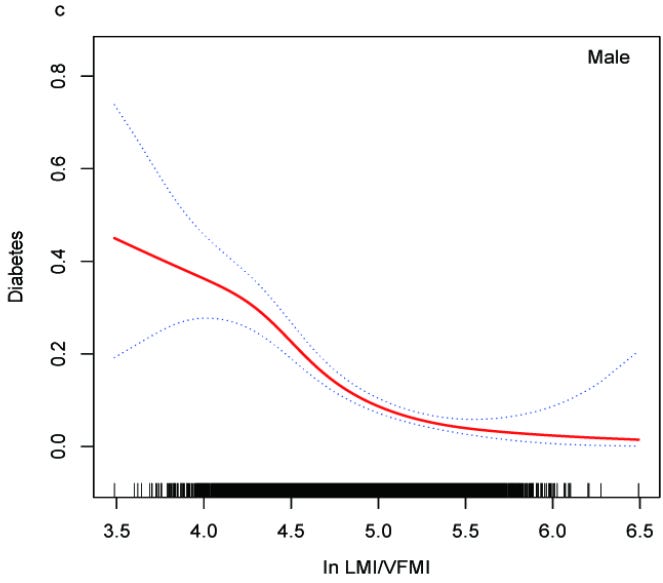
Higher LBM/VF ratios show strong inverse associations3 with dyslipidemia (A), elevated blood pressure (B), and insulin resistance (C)
This ratio becomes especially critical as you age. It captures sarcopenic obesity → the process where muscle wastes away & visceral fat accumulates simultaneously.
Optimal Ranges
**Note: All ranges are denoted in the natural logarithm format - “ln (X)” - due to the nature of the nonlinear relationship of ratio with odds ratio.
Optimal: ln(LBM/VFM) > 6.0
Good: ln(LBM/VFM) = 5.0 - 6.0
At Risk: ln(LBM/VFM) = 4.2 - 5.0
High Risk: ln(LBM/VFM) < 4.2
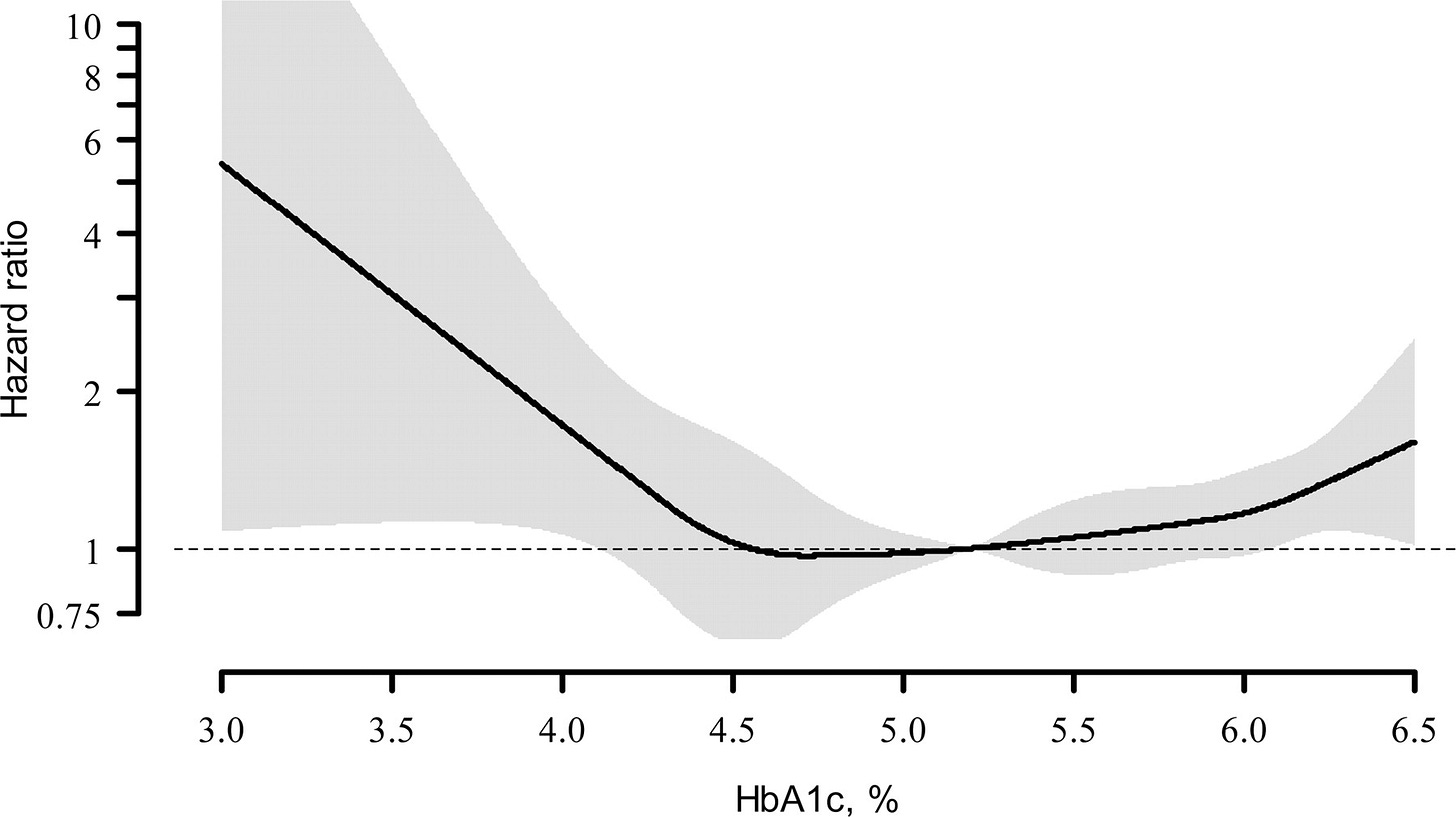
Biomarker #2: HbA1c
What it measures?
A 3-month average of blood glucose levels smoothing out the day-to-day variability seen with fasting glucose/postprandial glucose measurements.
Why it matters?
HbA1c serves as one of best biomarkers for metabolic flexibility → how efficiently you switch between burning glucose & fat for fuel. Optimal levels signal superior insulin sensitivity, robust stress responses, and enhanced ATP production. Given our metabolic health is so closely tied to cognitive health, HbA1c also represents a strong indicator of cognitive performance4 & white matter integrity in young adults.
A 2012 randomized controlled trial by Andersson et al.5 found for each 1% increase in HbA1c raised all-cause mortality risk by 12% in men and 22% in women. Similarly, a 2010 review by Carson et al.6 showed that an HbA1c of 6.0-6.4% relative to a 5.0-5.4% carried with a 28% increased mortality risk of all-cause mortality.
Optimal Ranges
Optimal: 4.6 - 5.3%
Good: 5.4 - 5.6%
Prediabetic: 5.7 - 6.4%
Diabetic: > 6.4%
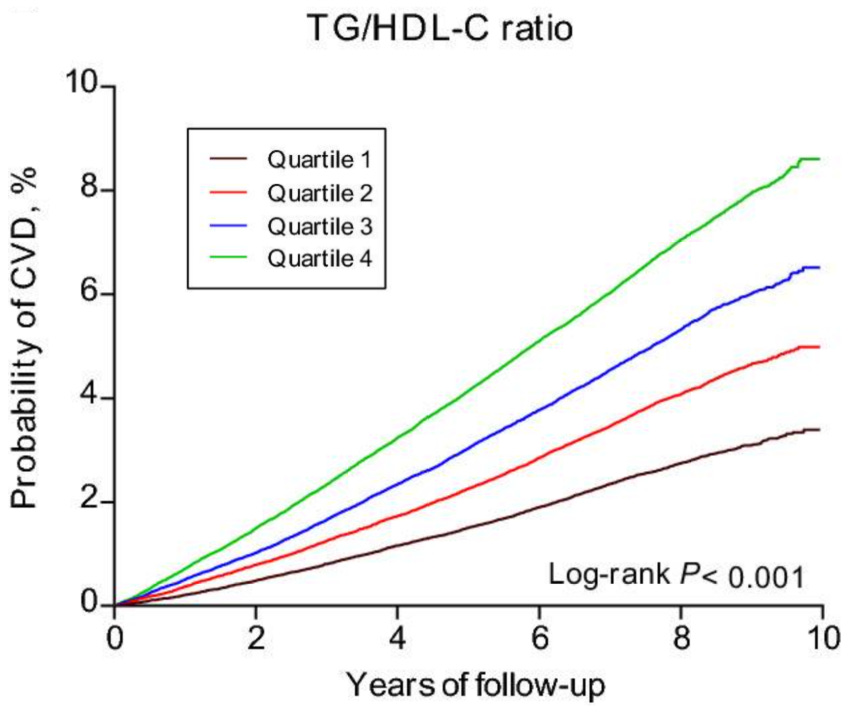
Biomarker #3: Triglyceride/HDL Ratio
What it measures?
Triglycerides: The body’s densest energy storage molecule. The primary fat form in adipose tissue & the dominant lipid in VLDLs.
HDL: Compact particles (50% protein + 50% lipid) that extract excess cholesterol from tissues (especially around arterial walls) and shuttle it to the liver for elimination.
Why it matters?
The TG/HDL ratio reveals cellular energy efficiency. An elevated ratio (high triglycerides + low HDL) signals suboptimal metabolic function: insulin resistance, chronic, low-grade inflammation, & mitochondrial dysfunction → all of which compromise the our ability to produce and utilize ATP efficiently at the cellular level.
Che et al. (2023)7 analyzed 400,000 subjects and found those in the highest TG/HDL quartile (5.5) faced 29% greater CVD risk versus the lowest quartile (1.1). This high-risk group showed higher rates of dyslipidemia (40%), hypertension (13.3%), & type 2 diabetes (11.8%).
Optimal Ranges
Optimal: < 1
Good: 1 - 2
At Risk: 2 - 3
High Risk: > 3
More on the triglyceride/HDL ratio:
Biomarker #4: ApoB/ApoA1 Ratio
What it measures?
The ApoB/ApoA1 ratio represents the balance between atherogenic & atheroprotective forces in the cardiovascular system.
ApoB: The primary structural protein found on all atherogenic (plaque-forming) lipoproteins, including VLDL, IDL, LDL, & Lp(a). Each atherogenic particle contains exactly one ApoB molecule, making it a direct particle count of all lipoproteins capable of penetrating the arterial wall & initiating atherosclerosis.
ApoA1: The main structural & functional protein component of HDL particles → comprising ~70% of HDL protein content. Each HDL particle typically contains 2-4 ApoA1 molecules. Functions as the primary activator of lecithin-cholesterol acyltransferase (LCAT) → the enzyme responsible for cholesterol esterification and reverse cholesterol transport from peripheral tissues back to the liver.
Why it matters?
The INTERHEART study8 - a landmark case-control analysis spanning 52 countries & ~30k participants - demonstrated that the ApoB/ApoA1 ratio was the single strongest lipid predictor of myocardial infarction risk. Individuals in the highest quintile (ratio >1.0) had a 3.25-fold increased risk of acute MI compared to the lowest quintile (ratio <0.6). ApoB/ApoA1 ratio outperformed all conventional lipid markers (i.e. total cholesterol, LDL-C, HDL-C, & triglycerides) in predicting cardiovascular events.
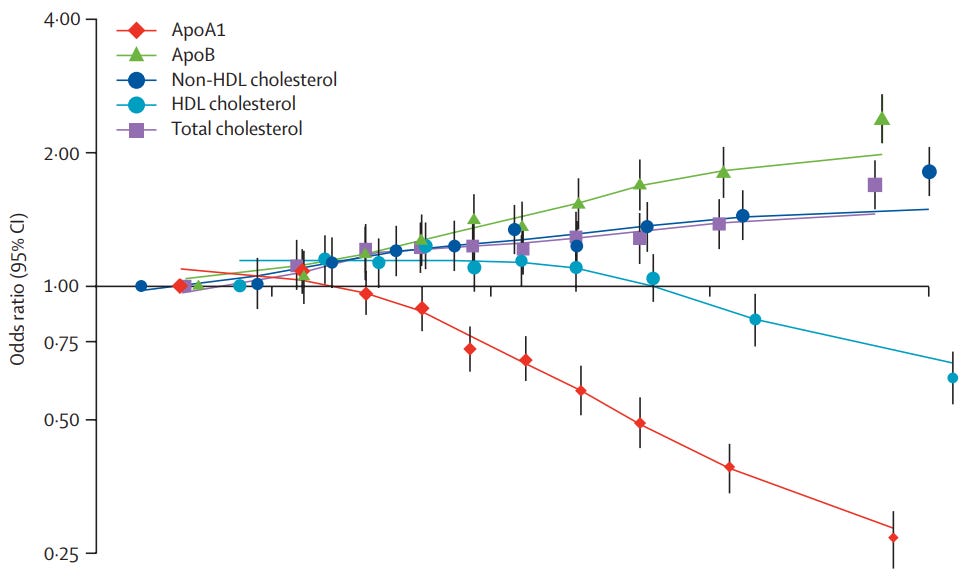
Optimal Ranges
Optimal: < 0.6
Good: 0.6 - 0.8
At Risk: 0.8 - 1.0
High Risk: > 1.0
Most PCPs stop at the basics.
Fasting blood glucose. Complete blood count. Maybe basic lipids. That’s it.
But if you actually care about your metabolic health, you need a complete picture → fasting insulin, ApoB, CRP, sex hormones, liver enzymes, & dozens of other markers that never make it into your standard blood draw order.
That’s why I use Superpower: a biomarker testing membership that runs 100+ markers in a single annual panel, builds you a personalized plan, & connects you with concierge-level medical practitioners.
Cost? $199.
Locations? 3,000+ labs nationwide.
Support? Unlimited access to 24/7 Concierge.
In the age of decentralized medicine, it’s the simplest way to take full control of your health & catch issues years before your PCP would.
The Superpower team loves the Phys audience so they’re dishing out this package at just $17/month with this link.
Test. Don’t guess.