The Molecule We’ve Been Ignoring in the Autism Conversation
Why taurine will be the next big brain health story
There’s a nutrient quietly shaping how the brain manages behavior. One most outside of this space have never thought twice about. And now, early evidence suggests that when levels of it drop in a specific brain region, we might see changes linked to neurodevelopmental disorders.
Last week on 𝕏 among many forecasts, we predicted taurine will become the “new creatine” in 2026.
So this week let me explain just one of the reasons why that's the case.
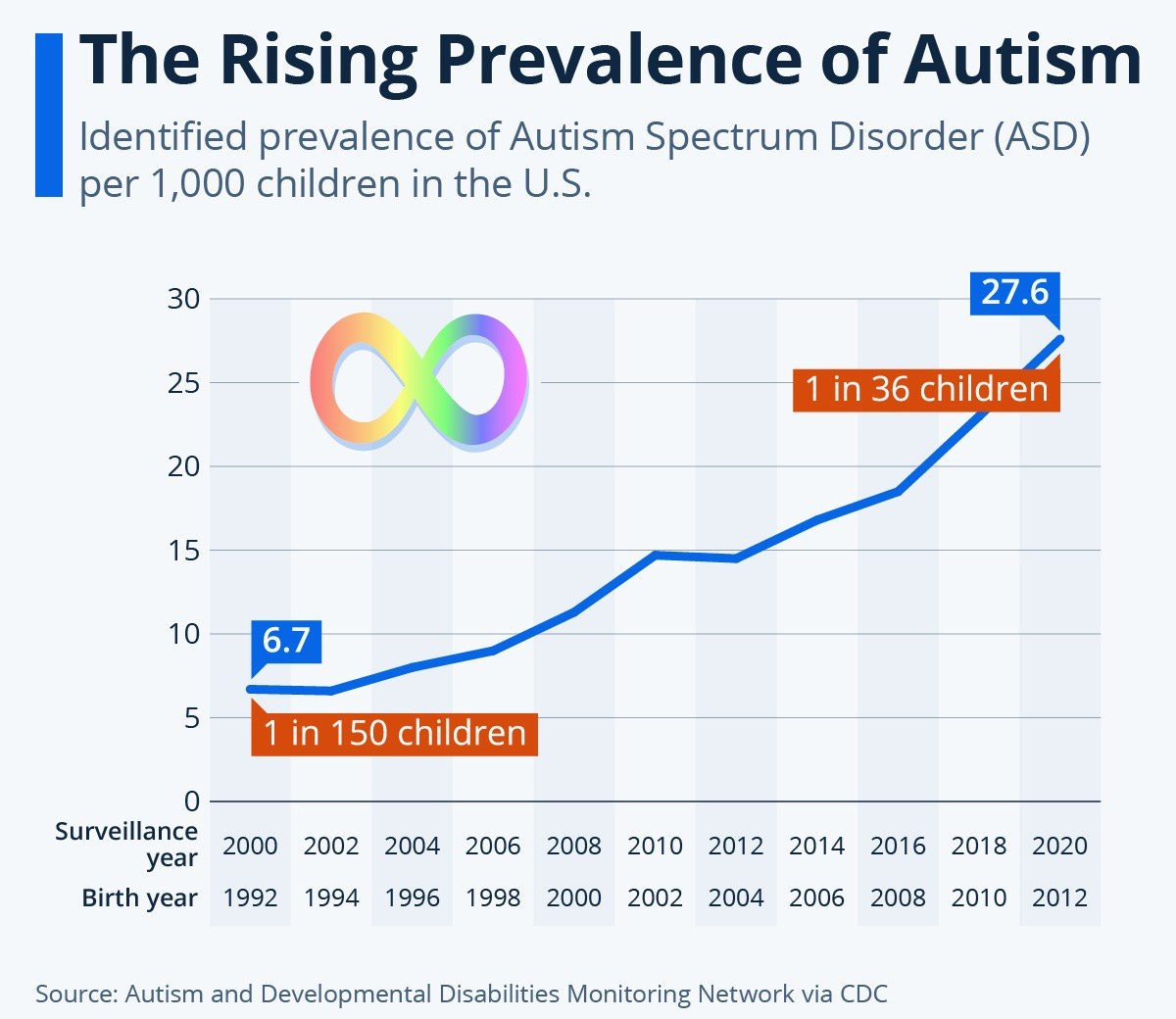
Autism now affects an estimated 1 in 36 children today and diagnoses continue to rise year over year.
The conversation is shifting from viewing autism only through the lens of behavior to understanding it biologically, metabolically, chemically.
Instead of asking “How do we manage symptoms?” the more interesting questions are becoming → “What’s happening inside the brain that drives these symptoms and can we support it?”
Oxidative stress is one leading theory. When we have redox imbalance runs, neurons don’t fire, communicate, or adapt efficiently. Glutathione usually dominates this discussion as the body’s main antioxidant defense system.
But there’s another molecule quietly sitting in the background: taurine. The amino-sulfonic acid with antioxidant, neuroprotective, & inhibitory signaling roles.
The Study
Researchers in Japan1 conducted the first in-vivo measurement of taurine levels directly in the brains of children with autism.
They asked two key questions:
Do taurine (Tau) & glutathione (GSH) levels differ in autistic vs neurotypical children?
If so — do those levels relate to real-world behaviors?
Specifically, they focused on what are called restricted & repetitive behaviors (RRBs). The loops, routines, & rituals often seen in autism. Parents & clinicians know them well, but we’ve faded them biologically.
Study Snapshot
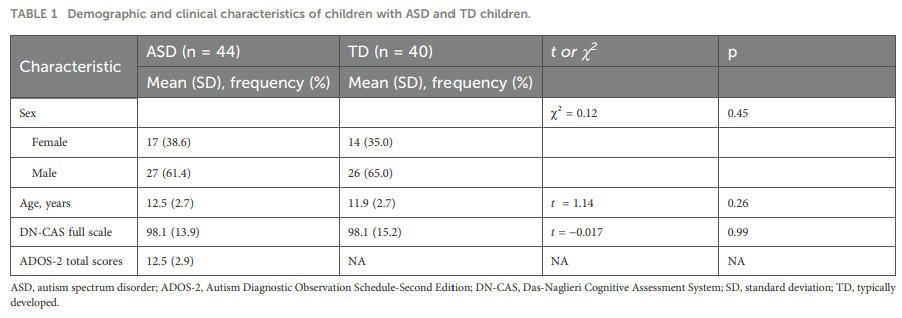
Who were the subjects?
44 children with autism (ASD)
40 typically developing controls
Age range: 8–16 years
Cognitive levels matched
What did researchers study?
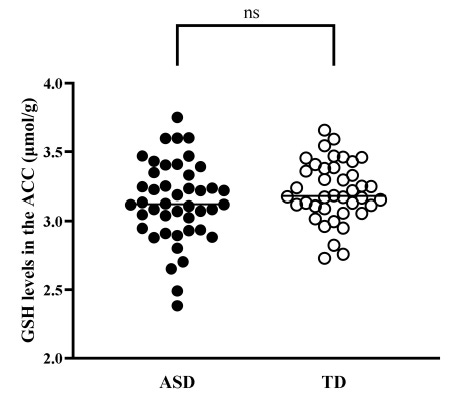
Using advanced magnetic resonance spectroscopy (MRS), they measured taurine & glutathione concentrations in a specific brain region: the anterior cingulate cortex (ACC).
Why the ACC?
Previous neuroimaging work has linked ACC differences to repetitive behaviors in autism. And because it’s heavily involved in behavior regulation, cognitive flexibility, & impulse control.
What outcomes were measured?
Taurine levels (Tau)
Glutathione (GSH)
Behavioral scores
The Findings
This is where it gets interesting.
Taurine was significantly lower in autistic children.
Autism average taurine: 4.48 mmol/g
Typical development average taurine: 5.08 mmol/g
p = 0.001 (highly significant)
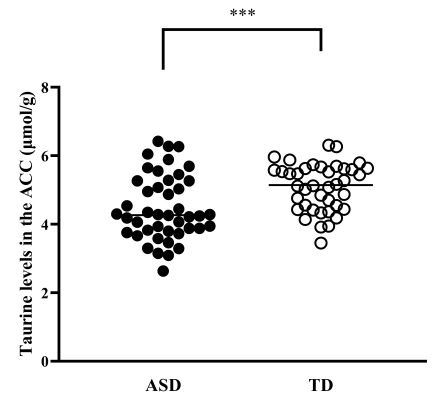
This difference held even after removing children on medication or with comorbid conditions.
Glutathione levels were the same in both groups.
This is surprising considering many past studies link oxidative stress to autism2. Yet here, glutathione looked normal.
So taurine dropped & glutathione didn’t….an asymmetry hinting at distinct biological pathways at play.
Lower taurine correlated with more severe repetitive behaviors.
The lower the taurine level = the more pronounced the repetitive behaviors.
Why Would Taurine Matter?
Taurine is more than an amino acid:
fuels mitochondrial energy production
modulates inhibitory neurotransmission (calming signal gain)
protects cell membranes from oxidative damage
influences GABA/glycine signaling during brain development
Consider taurine as a neurochemical coolant. When chronic stress & inflammation increase, taurine is there to mitigate “overheating”.
The Loop:
Taurine ↓ → Reduced inhibitory signaling → Oxidative stress ↑ → More taurine depletion → ACC dysregulation → Repetitive behaviors ↑
Layer on top many autistic children have selective eating patterns3 & higher urinary taurine excretion4 and now we’re getting somewhere on the more complete biological story.
Practical Takeaways
Taurine is an underrecognized molecule in neurodevelopment.
When we say taurine is the new creatine, we mean that in a couple ways. First, in terms of popularity. Its virtually unheard of outside of our side of the health space. And maybe a few people that bothered to read a Red Bull can before. And second, in terms of its neuroprotective potential.
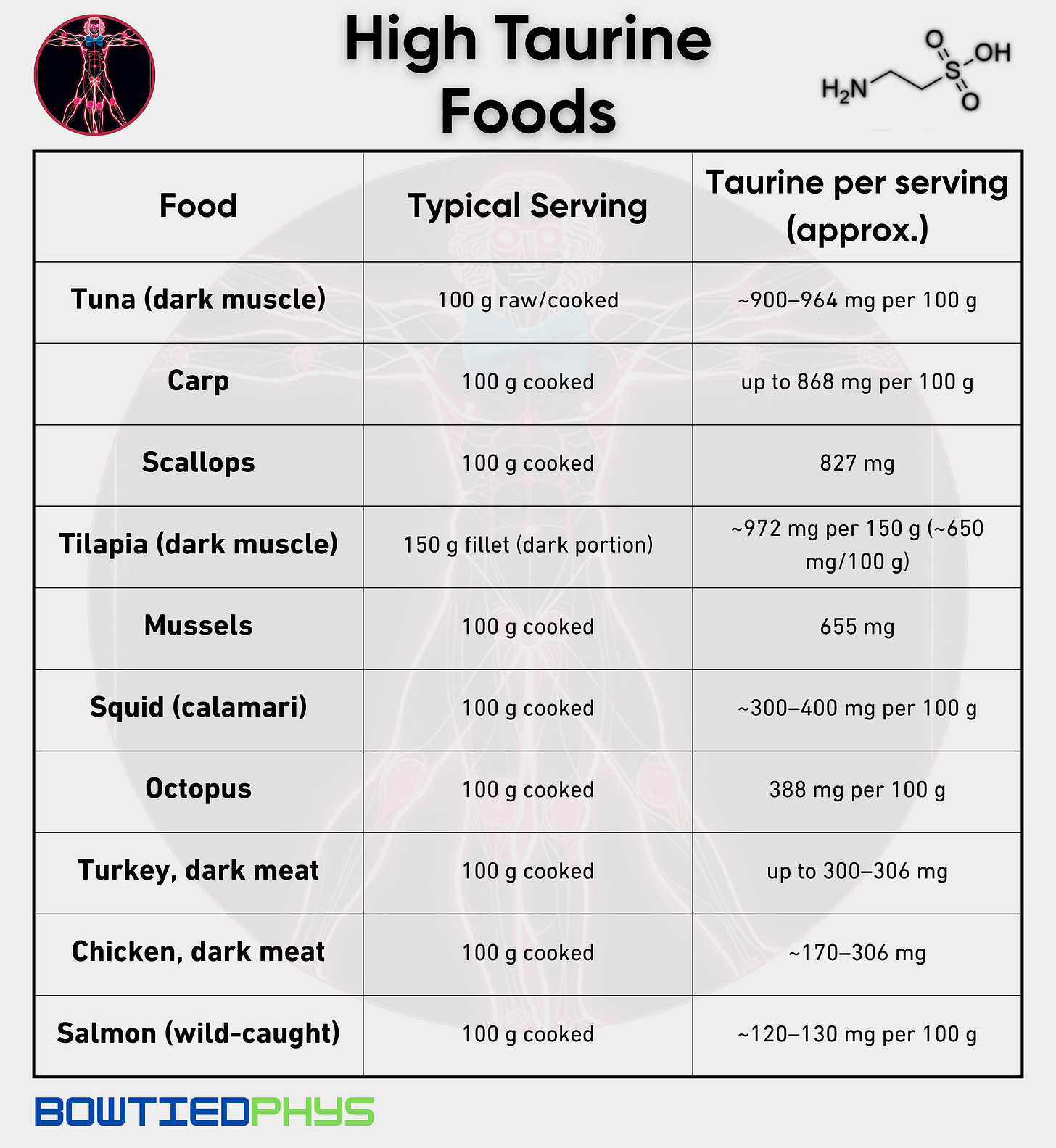
Nutrition first (seafood + dark meats). Supplementation last. But not supplementation never.
Seafood. Dark meats. Then targeted supplementation.
Research momentum is building.
One of the studies I’m most looking forward to in 2026: A randomized clinical trial on taurine supplementation in autistic children5. Already registered & underway. We’ll be watching this closely.
If for nothing else — we’re bullish on the treatment of nutrients as critical components to behavior.
The brain is electrical. The brain is emotional. The brain is chemical. And sometimes the biggest breakthroughs in this space come not from new pharmaceuticals, but from restoring balance to a single molecule.
If taurine is a part of that equation, we’re standing at the early edge of a very exciting chapter to come. Next few years are going to be fun in this space.
See you guys next week.
Phys
Minami A, Matsuoka K, Takahashi M, Ueda K, Ohnishi H, Fujimoto Y, Yoshikawa H, Ishida R, Takado Y, Near J, Yamatani Y, Miyasaka T, Tai Y, Ochi T, Tanaka T, Okada T, Iwata N, Makinodan M. Association between decreased taurine levels in the anterior cingulate cortex and restricted and repetitive behaviors in autism spectrum disorder: a cross-sectional study. Front Psychiatry. 2025 Dec 11;16:1700059. doi: 10.3389/fpsyt.2025.1700059. PMID: 41458030; PMCID: PMC12739181.
Liu X, Lin J, Zhang H, Khan NU, Zhang J, Tang X, Cao X, Shen L. Oxidative Stress in Autism Spectrum Disorder-Current Progress of Mechanisms and Biomarkers. Front Psychiatry. 2022 Mar 1;13:813304. doi: 10.3389/fpsyt.2022.813304. PMID: 35299821; PMCID: PMC8921264.
Cermak SA, Curtin C, Bandini LG. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J Am Diet Assoc. 2010 Feb;110(2):238-46. doi: 10.1016/j.jada.2009.10.032. PMID: 20102851; PMCID: PMC3601920.
Nadal-Desbarats L, Aïdoud N, Emond P, Blasco H, Filipiak I, Sarda P, Bonnet-Brilhault F, Mavel S, Andres CR. Combined 1H-NMR and 1H-13C HSQC-NMR to improve urinary screening in autism spectrum disorders. Analyst. 2014 Jul 7;139(13):3460-8. doi: 10.1039/c4an00552j. PMID: 24841505.
Chen Y, He W, Deng Q, Peng Z, Tai Z, Ma Y, Wang T, Wang Y, Yan W, Zhou H. Taurine supplementation in children with autism spectrum disorders: a study protocol for an exploratory randomized, double-blind, placebo-controlled trial. BMC Pediatr. 2025 Oct 27;25(1):871. doi: 10.1186/s12887-025-06216-0. PMID: 41146076; PMCID: PMC12560396.




Really thoughtful piece. As a clinician scientist, I love seeing the autism conversation move from “behavior only” to biology + behavior, without reducing a heterogeneous condition to a single pathway. The taurine signal here is especially intriguing because it’s not just an “antioxidant” story: taurine touches inhibitory tone (GABA/glycine modulation), neurodevelopmental signaling, mitochondrial energetics, and even osmoregulation; exactly the kinds of fundamentals you’d expect to influence circuits like the ACC that govern cognitive flexibility and repetitive behaviors. That said, this is still an association (cross-sectional MRS), so the key question becomes: marker, mediator, or consequence? Diet selectivity, sleep/circadian disruption, inflammation, meds, and broader metabolic context could all push taurine and symptoms in the same direction. The registered RCT you mention is the right next step, and I’m very curious whether responders cluster by phenotype (diet pattern, GI features, baseline taurine, sensory profiles, etc.). Practical take-home I’d echo: food-first (seafood/dark meats if appropriate), caution against “miracle molecule” framing, and keep supplementation firmly in the “adjunct/under study” bucket, not a replacement for established supports. If taurine ends up being a modifiable lever for a subset, that would be a genuinely meaningful win.