The Hidden Deficiencies Sabotaging Your Carb Metabolism (Part 1)
The 10 methods to unf*ck your glucose metabolism using light, time, & environment
Time to put to rest the long-standing vilification of an entire macronutrient. Contrary to dogmatic schools of nutrition in this space, the real culprit behind metabolic dysfunction isn't carbohydrates themselves. Rather a set of critical deficiencies in how we support our body's natural energetic processing systems. This week we start to build our framework based on this idea.
Yes - it's highly manipulatable with a robust default system in place. When these foundational elements are in place, our body becomes remarkably efficient at not just handling carbs, but leveraging them for peak physical performance & stacking on more of our most critical longevity organ - skeletal muscle.
Here are the 10 highest ROI tools to dial in your carbohydrate metabolism.
Deficiency #1: Red & Infrared Light Wavelengths
0.072% of our human existence has been spent under this new age phenomenon called artificial light. For 200,000 years, we (& our metabolic processes) evolved under full-spectrum solar exposure. It wasn’t until 1879 with the invention of the electric light that our relationship with light as a species began to drastically pivot. And now today we spend a majority of our time (93%) indoors. Obvious benefits here, but it doesn’t come without a cost.
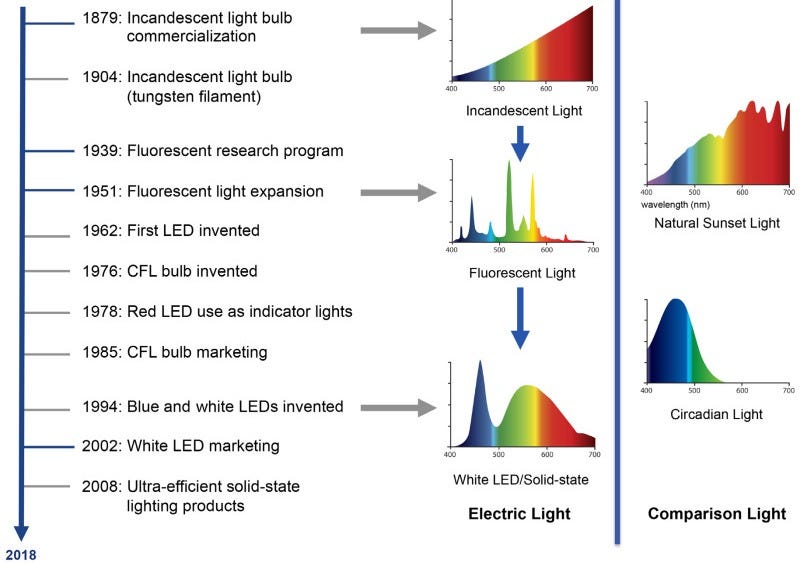
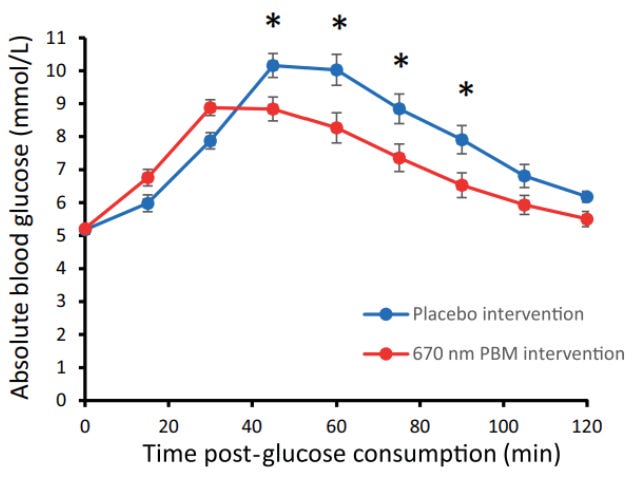
Red light wavelengths ~620-750 nm & near-infrared wavelengths ~750-2500 nm play crucial roles in driving mitochondrial bioenergetics involved in our ability to metabolize carbs. These bands of light enhance mitochondrial function: 1) electron transport chain activity, 2) mitochondrial membrane potential, & 3) ATP production. When we’re deficient in these bands of light, our cells have a reduced demand for glucose and therefore more glucose remains in circulation causing greater glycemic areas under the curve & maximum spikes.
In early 2024, Powner et al. demonstrated the best clinical example to date we have of this principle. They found 15 min of exposure to 670 nm light reduced blood glucose elevation by 28% over two hours after initial glucose intake with the peak glucose spike reduced by 7.5%.
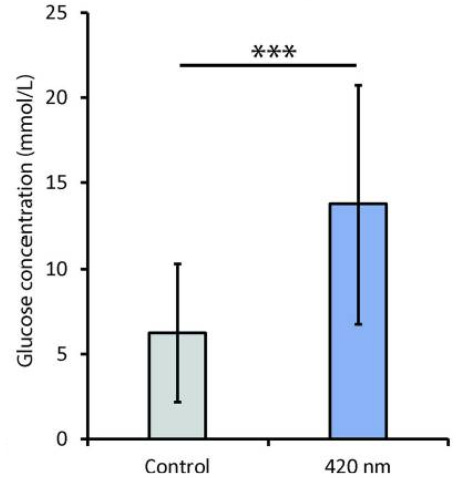
But not all wavelengths of light are equal in their impacts on our mitochondrial metabolism. In fact, shorter wavelengths ~420 nm (i.e. blue light) inhibit mitochondrial respiration increasing blood glucose levels by more than 50% in animal models. Consider this the next time you beam yourself with a blue-lit screen at your next meal.
Deficiency #2: Slow-Wave Sleep (10 PM - 2 AM)
“It’s only one night. Not that big of a deal.”
Just a single night of minimal deep non-REM sleep disrupts glucose metabolism independent of total sleep time.
In 2013, Herzog et al. demonstrated this when sixteen subjects were placed in three different sleep conditions: one night of acoustic disturbance of slow-wave sleep (dashed & dotted), REM-sleep (dotted), or regular sleep (solid). Relative to REM & regular sleep disturbances, the slow-wave disturbance reduced insulin sensitivity by 20% & 15%, respectively.
As we’d expect from reduced insulin response, the area under the glucose curve was also significantly greater for the slow-wave suppression than the other two sleep disturbance conditions.
Why we’ll continue to emphasize quality & regularity of sleep cycles remain more critical than quantity of sleep alone.
Deficiency #3: GH + IGF-1
It shouldn’t come as a surprise a lack of endogenous growth hormone is closely linked to poor glucose metabolism.
Why?
Our strongest pulse of growth hormone occurs during our time in a deep sleep state and serves as the primary driver of why slow-wave sleep suppression dysregulates insulin signaling.
See how we’re connecting the dots here…
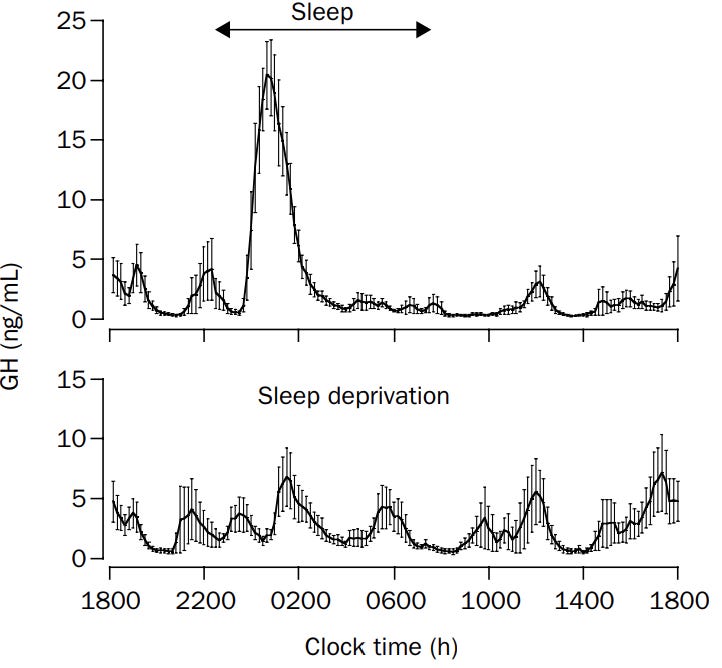
A lack of GH secretion leads to two big impacts for us: 1) a poor ability to oxidize fat & 2) worsened metabolic flexibility. GH stimulates the fat-burning process resulting in the mobilization of fatty acids from adipose tissue to circulation. With less GH, we get less breakdown of fat.
As a result of downregulated GH production, we get lower levels of IGF-1 as well. Thus, leading to less glucose uptake by the muscles & liver and an impaired ability to maintain glucose homeostasis over time.
Deficiency #4: Mitochondria
At the root of every one of our deficiencies outlined hereafter is a common culprit: mitochondrial dysfunction.
Where the deficiency comes into play is when PGC-1α (master regulator of mitochondrial biogenesis) starts to decline significantly. Without consistent activation of this pathway, damaged mitochondria are left unreplaced creating a decline in mitochondrial capacity.
When mitochondria can't produce an adequate amount of our bodies’ energy currency (ATP), our cells struggle to perform glucose-dependent functions. This is why we see ~30% lower mitochondrial oxidative capacity in muscle tissue of insulin-resistant individuals.
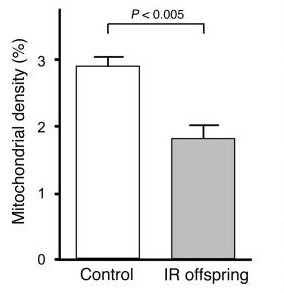
There’s even an epigenetic (highly mutable) component involved in our mitochondrial makeup. Morino et al. reported the rate of insulin-stimulated muscle glucose uptake as 60% lower & muscle mitochondrial density ~40% lower in the children of insulin resistant parents than the children of metabolically healthy parents.
Deficiency #5: Parasympathetic Activation
When our parasympathetic system is chronically under activated & our sympathetic system is in overdrive, signaling between our brain and key metabolic organs like the pancreas, liver, & fat tissue becomes disrupted.
In the pancreas, parasympathetic signals release acetylcholine triggering the release of insulin. However, when we’re lacking that tone, the insulin responses become blunted and lead to elevated blood glucose levels and eventual glucose intolerance. Similarly, the liver requires parasympathetic activation to suppress glucose production (via gluconeogenesis) while promoting glucose storage as glycogen. Without it, the liver keeps spamming glucose into circulation.
This is why people with lower heart rate variability consistently show higher insulin resistance, worse glucose tolerance, & an increased risk of developing diabetes.
Deficiency #6: Muscle
Skeletal muscle isn’t just a glucose sink - it is the glucose sink. As the largest organ system by mass, our muscle mass serves as the predominant glucose disposal tissue accounting for ~80% of postprandial glucose uptake.
Part of the mechanism is due to having less tissue to store glucose. But another primary driver for this lies in the concept of GLUT4 expression. These transport proteins act like doors opening in response to insulin to let glucose into each muscle cell. When we lose muscle mass, we also lose these GLUT4 transporters proportionally and the insulin receptors that signal these doors to open. As a result, we have fewer entry points for glucose and weaker signals to open them.
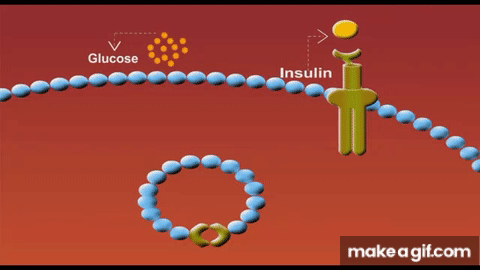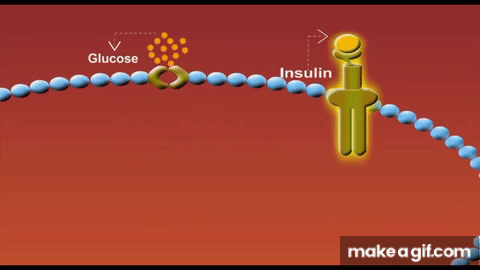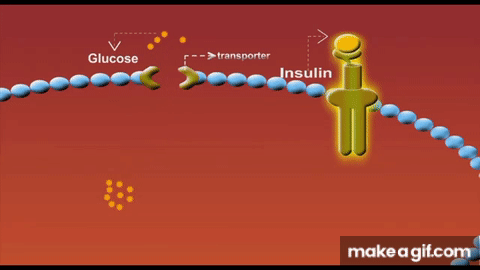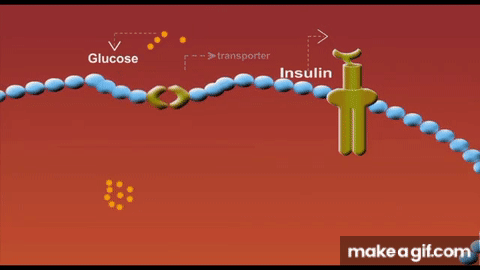
Solution to defeat sarcopenia-induced insulin resistance? Lift weights → stack muscle mass. Simple.
Deficiency #7: HIIT
If there was a king of insulin sensitization, the crown would belong to high-intensity interval training (HIIT).
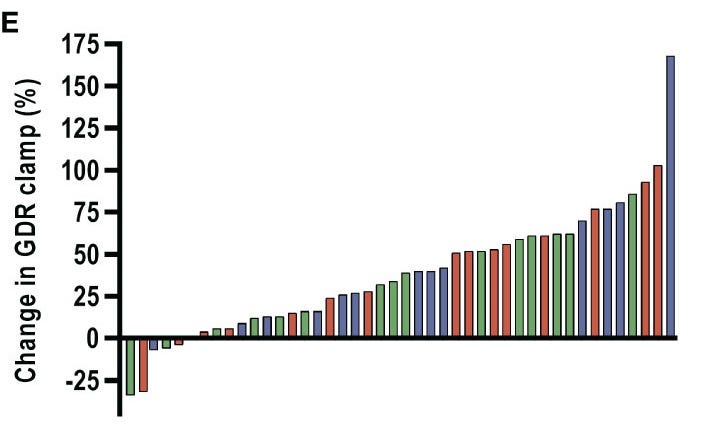
A 2022 trial by Petersen et al. showed that in an 8-week HIIT protocol, insulin-stimulated glucose disposal rates significantly increased by 42% in type 2 diabetics, 27% in obese men, & 29% in lean men.
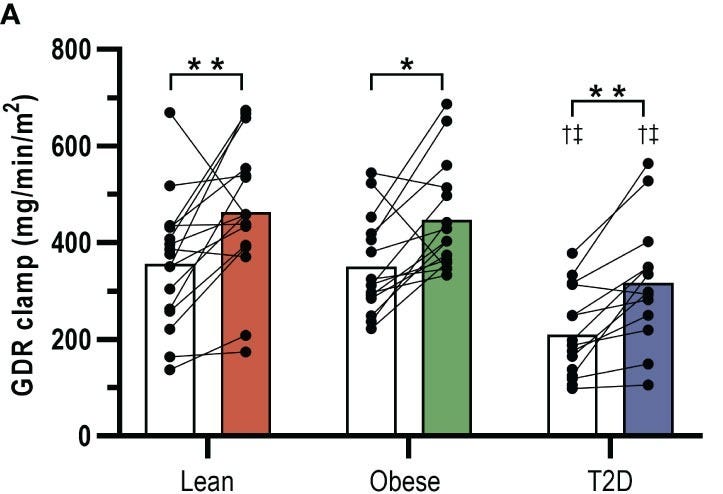
Why HIIT is so powerful hinges upon glycogen depletion & mitochondrial remodeling in our fast-twitch muscles. Quick sprints exhaust glycogen stores in type II fibers creating a metabolic demand driving glucose uptake for hours after exercise as muscles prioritize a refuel phase. Simultaneously, the metabolic stress from the max pain activates PGC-1α (there it is…again) increasing mitochondrial density.
The rapid impacts are about as close to a physiological miracle as we see in the health space. Sjöros et al. demonstrated just 6 sessions over a 2-week period normalized muscle glucose uptake in diabetics. This kind of response was ~2x faster than typical Zone 2/3 moderate-intensity training.
More intense HIIT → more mitochondria → improved glucose response
Deficiency #8: Melatonin
The mainstream narrative of melatonin as the “sleep hormone” has largely missed the point. More than 95% of our melatonin is actually made inside our mitochondria where it acts as an antioxidant influencing glucose metabolism.
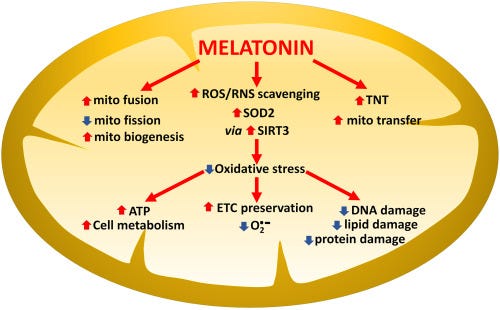
Without sufficient melatonin protection, mitochondria suffer oxidative damage to their electron transport chain causing “electron leakage” and creating free radicals, which further impair the mitochondria's ability to efficiently process glucose into ATP.
Given the mechanisms outlined above it shouldn’t be surprising that lower melatonin secretion is tightly associated with a higher risk of developing type 2 diabetes over time.
Deficiency #9: Postprandial Movement
When we move after eating, our muscles become highly efficient glucose disposal units. The mechanism of movement after a meal is so powerful it can increase muscle glucose uptake ~2x more than at rest.
Without post-meal movement, liver glucose production remains 0.3-0.5 mg/kg/min higher than it would be having moved during the 2-4 hours after eating. This translates to an extra 15-20 mg/dL of glucose in our bloodstream.
“How long after a meal should I move?”
Peak glucose concentrations occur 60-90 minutes post-meal in healthy individuals and 90-120 minutes in those with glucose intolerance. Therefore, the sweet spot to begin a 10-20 minute bout of movement is 40-50 min after meal completion. Though, worry less about the optimal timing. And more about adopting the act as a habit.
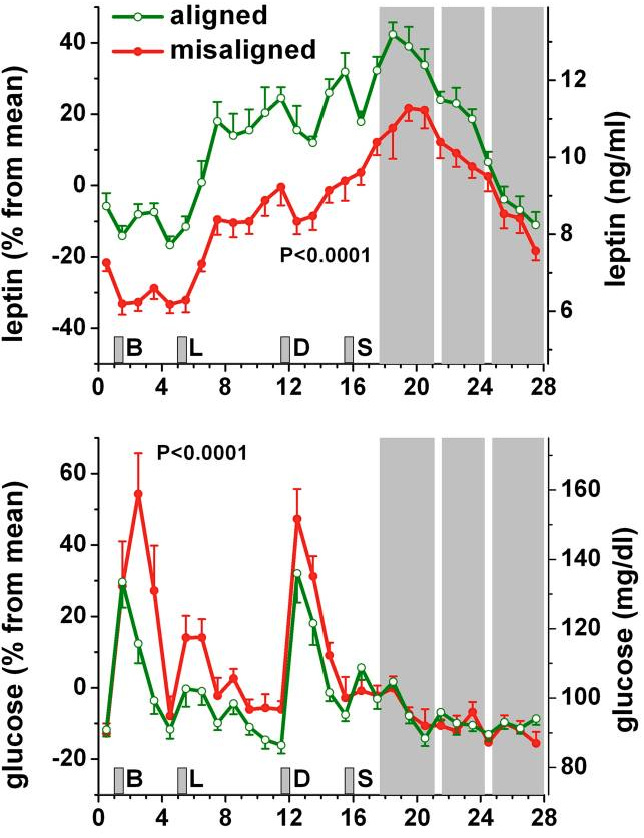
Deficiency #10: Circadian Alignment & Meal Timing
Expect the area of chrononutrition to become even more popular in the coming year. The verdict is now out on light & time, while the next foundational pillar to be planted will be energy intake & time.
Our bodies expect consistency across any given day. It’s part of the reason why postprandial responses are contingent on meal timing and it further explains why perpetual circadian misalignment contributes to harsh metabolic & cardiovascular implications - suppression of leptin levels & elevated glucose responses.
Downstream of circadian regulation resides the phenomenon of consistent timing of nutritional intake. Even 5-hr delays in meal timing can shift glucose & insulin response rhythms by almost 6 hrs disrupting hormonal signaling for multiple days independent of sleep duration.
The circadian-aligned meal protocol is simple: Big breakfast. Medium-sized lunch. Light dinner. Consistently-timed each day.
Alright that’s a wrap on our ten deficiencies.
“Phys - all these levers mentioned and no single mention of nutrition?”
Way more on our nutritional deficiencies in Part 2 next week. Until then, stay after it & we’ll see you next week.
Your friend,
Phys
***Disclaimer: The information provided by BowTiedPhys is for educational purposes only. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment of any kind. BowTiedPhys is not a licensed medical provider. Prior to making any changes to your health protocols, consult a licensed healthcare professional. Some of the links in this post may be affiliate links - BowTiedPhys may earn a commission at no additional cost to you. This commission helps support our work and allows us to continue providing valuable content to our valued subscription members.***